ConductScience Digital Health





Capture symptons, emotions, thoughts & sensations
Capture physical metrics, functions, habits & activities
Utilize mobile technology in groundbreaking ways
Implement, integrate w EMR, Branding & Monetization

ConductScience makes it easy to create the tools you need to track your patient’s data, analyze, and export. Your app intervention, your data. We do the work. You get published.

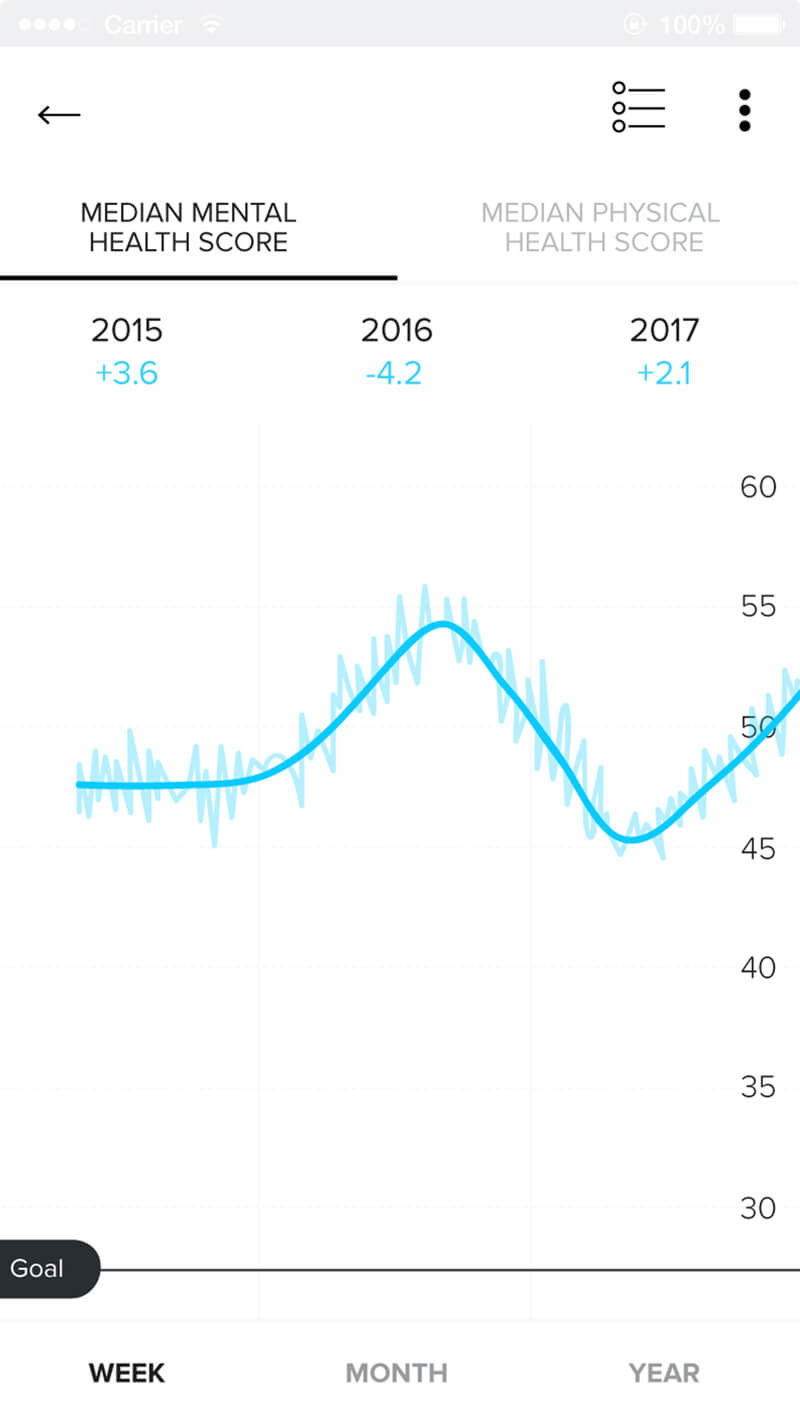
Rich Patient Data

As subcontracted developers, data generated in your app developed through ConductScience will always be yours, in right to publish, monetize, and create.

Conduct Science has experienced digital health developers for a wide array of custom possibilities for your study.

We’ll take care of the headaches. We’ll schedule meetings, nudge you on periodic check-ins and feedback, and help manage your study and technology.

ConductScience comes with optional Tech Transfer options, to monetize and bring your digital health tool to market in ways never before possible. You did the research, you should benefit from your work.

Have questions? Ask anything!
Contact Us
"*" indicates required fields
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.